18+ Calculate The Maximum Concentration In M Of Silver Ions
Given E AgAg 080 V E Cu2Cu 034 V Answer. Long Answer Type Questions 5 Marks 87.

Ijerph December 2 2021 Browse Articles
To this solution is added 500 mL of 150 M hydrochloric acid and a precipitate forms.

. A sample of gallium bromide GaBr 3 weighing 0165 g was dissolved in water and treated with silver nitrate AgNO 3 resulting in the precipitation of 0299 g AgBr. Electron mobility is almost always specified in units of cm 2 VsThis is different from the SI unit of mobility m 2 VsThey are related by 1 m 2 Vs 10 4 cm 2 Vs. One can calculate the amount of AgCl that will dissolve in 1 liter of.
6 to 30 characters long. The copper ion concentration is 010 M. Calcium carbonate is a chemical compound with the formula Ca CO 3It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock consisting mainly of calcite and is the main component of eggshells gastropod shells shellfish skeletons and pearlsCalcium carbonate is the active ingredient in agricultural.
Moreover fewer than four in ten across regions gender racialethnic education and income groups would vote yes Likely voters ages 18 to 44 41 are far more likely than older likely voters ages 45 and above 19 to say they would vote yes. Determine the concentration of silver ions in the cell. Does a sodium hydroxide solution have a suitable concentration if titration of 1200 mL of the solution requires 306 mL of 165 M HCI to reach the end point.
Microsoft pleaded for its deal on the day of the Phase 2 decision last month but now the gloves are well and truly off. The pH scale is logarithmic and inversely indicates the. Video 18 min U.
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference as a measurable and quantitative phenomenon and identifiable chemical change with the potential difference as an outcome of a particular chemical change or vice versaThese reactions involve electrons moving via an electronically-conducting phase. The presence or absence of a compound is determined by a qualitative analysis but not the mass or concentration. 140 g of silver nitrate is dissolved in 125 mL of water.
A copper-silver cell is set up. Conductivity is proportional to the product of mobility and carrier concentration. Stanley Whittingham in 1974 who first used titanium disulfide TiS 2 as a cathode material which has a layered structure that.
Flinn Scientific is the 1 source for science supplies and equipment both in and outside the classroom. Each paper writer passes a series of grammar and. However there are two NH 4 ions per formula unit so the concentration of NH 4 ions is 2 143 M 286 M.
Because each formula unit of NH 4 2 Cr 2 O 7 produces three ions when dissolved in water 2NH 4 1Cr 2 O 7 2 the total concentration of ions in. Microsoft describes the CMAs concerns as misplaced and says that. Must contain at least 4 different symbols.
Our Custom Essay Writing Service Features. 1 serial dilutions are prepared. ASCII characters only characters found on a standard US keyboard.
Carbon hydrogen oxygen nitrogen phosphorus sulphur and halogens are the elements that make up organic molecules. K sp 18 10 10 from a table of solubility products Ag Cl in the absence of other silver or chloride salts so Ag 2 18 10 10 M 2 Ag. The concentration of silver ion is not known.
Solid solubility refers to the maximum concentration of impurities one can place into the substrate. For more than 40 years Flinn has been the Safer Source for Science. The ratio for pH values less than 65 was calculated by dividing the lower guideline 65 by the concentration measured pH.
What is the concentration in units of moiarity of silver ions remaining in the solution. In chemistry pH p iː ˈ eɪ tʃ historically denoting potential of hydrogen or power of hydrogen is a scale used to specify the acidity or basicity of an aqueous solutionAcidic solutions solutions with higher concentrations of H ions are measured to have lower pH values than basic or alkaline solutions. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing.
The breakthrough that produced the earliest form of the modern Li-ion battery was made by British chemist M. For example the same conductivity could come from a small number of electrons with high mobility for each or a large. The cell potential when measured was 0422 V.
However there are two NH 4 ions per formula unit so the concentration of NH 4 ions is 2 143 M 286 M. Deuterium or hydrogen-2 symbol 2 H or deuterium also known as heavy hydrogen is one of two stable isotopes of hydrogen the other being protium or hydrogen-1The nucleus of a deuterium atom called a deuteron contains one proton and one neutron whereas the far more common protium has no neutrons in the nucleusDeuterium has a natural abundance in. The ratio for dissolved oxygen was calculated by dividing the guideline by the concentration.
PH measurements must lie within a range of generally 65 and 9. Qualitative analysis by definition do not measure quantity. Solutions are initially solubilized in water and diluted in DMEM containing 05 fetal bovine serum FBS 05 FBS is the maintenance level required for HDF growth to give a final concentration of 100 g L.
For panel b E h 0 was obtained or calculated from the standard Gibbs free energy of formation ΔG f 0 from refs 186187188 and converted to E h as a function of Fe 2 concentration at. Today fewer than three in ten across partisan groups would vote yes on Prop 27. Because each formula unit of NH 4 2 Cr 2 O 7 produces three ions when dissolved in water 2NH 4 1Cr 2 O 7 2 the total concentration of ions in.
Following a bumpy launch week that saw frequent server trouble and bloated player queues Blizzard has announced that over 25 million Overwatch 2 players have logged on in its first 10 daysSinc. One of the earliest examples is a CuF 2 Li battery developed by NASA in 1965. Research on rechargeable Li-ion batteries dates to the 1960s.
The projector augmented wave pseudopotentials 3132 were used to calculate the interaction between ions and electrons in a plane wave basis set with a cut-off energy of 500 eV and a 5 5 1. Solutions are filtered through a 02 M sterile filter prior to addition to the cells and 1.

When Equal Volumes Of The Following Solutions Are Mixed Precipitation Of Agcl Ksp 1 8 10 11 Will Occur Only With

Solved Hey I Need Help With Finding The Molarity Of Unknown Chegg Com

Pdf Advices For Studying Organic Chemistry Sabrina Islam Academia Edu

Tablas Cineticas Pdf Pdf Ether Hydrogen
First Document Gsi
Solved 5 Calculate The Maximum Concentration In M Of Silver Ions Ag In A Solution That Contains 0 00125 M Of Co32 The Ksp Of Ag2co3 Is 8 46 10 12

Mic A And C And Mbc B And D Values Of Silver Ions Ag And Download Scientific Diagram

What Is The Maximum Concentration Of Ag That Can Be Added To A 0 00730 M Solution Of Na Co Before A Precipitate Will Form Ksp For Ag Co Is 8 10 10 Wyzant Ask An Expert
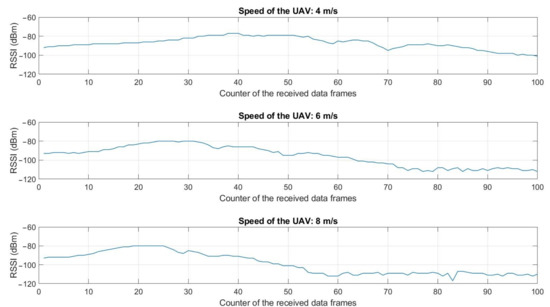
Sensors August 1 2022 Browse Articles
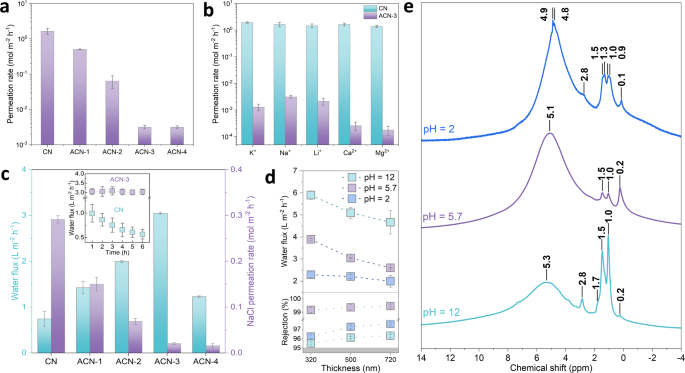
Lamellar Carbon Nitride Membrane For Enhanced Ion Sieving And Water Desalination Nature Communications
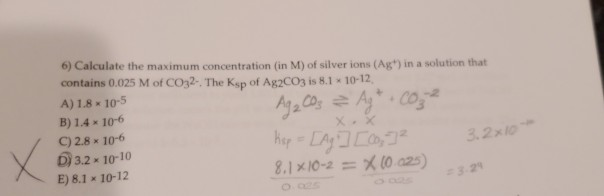
Solved 6 Calculate The Maximum Concentration In M Of Chegg Com

Solved U Agbiz 5 Calculate The Maximum Concentration In Chegg Com
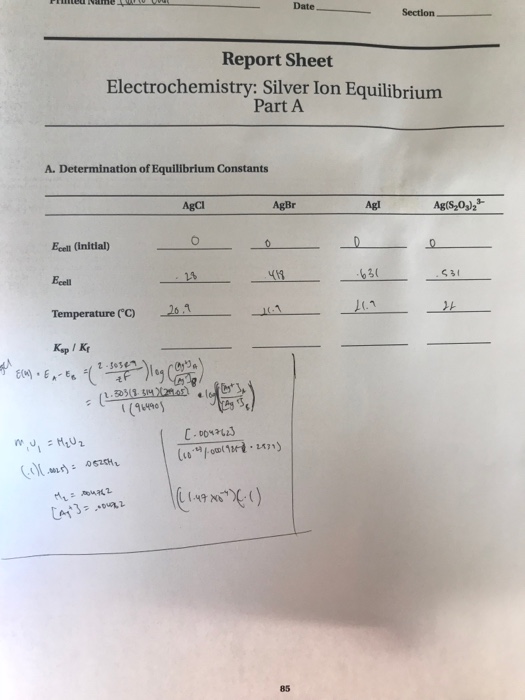
Solved Report Sheet Electrochemistry Silver Ion Equilibrium Chegg Com

The Aqueous Solution Of Which Of The Following Sulphides Contain Maximum Concentration Of S 2 Ions

Solved 45 Calculate The Maximum Concentration In M Of Silver Ions Agt In A Solution That Contains 0 025 M Of Co32 The Ksp Of Ag2co3 Is 8 10 12 A 1 8 10 5 B 1 4 10 6 C 2 8 10 6 D 3 2 10 10 E 8 1 10 12

Pdf The Mobility Of Silver Nanoparticles And Silver Ions In The Soil Plant System

Mechanisms Of Nucleation And Growth Of Nanoparticles In Solution Chemical Reviews